Locus coeruleus projects
We established the first-ever simultaneous recordings of many brainstem locus coeruleus (LC) neurons and are working with a team of international collaborators to develop large-scale brainstem neuronal recordings using ultra-flexible multi-electrode probes. Discover more about our locus coeruleus projects below.
Are the activity patterns of LC neurons conserved by evolution?
The brainstem is full of different collections of cells - all evolutionarily ancient and many controlling basic life-sustaining functions. It makes sense that, across species, these neurons have similar activity patterns. For instance, in mammals, phenomena as basic as breathing are driven by brainstem activity patterns that remain consistent across individuals – it does not matter whether they are male or female, young or old, asleep or awake - the brain activity driving breathing is the same. But is this true for the ancient locus coeruleus?
Given that the locus coeruleus has been conserved across vertebrates, it is possible that 1) the area’s activity is inherently similar across species, and 2) age, sex, and brain state influence locus coeruleus activity similarly across species.
Dr. Totah led a consortium of 20 laboratories from 10 countries to find out if the activity of the locus coeruleus is truly conserved. Remarkably, the consortium found that activity differed due to complex interactions of species, sex, and brain state.
Our work raises new questions about how this ancestral region may contribute to sex, species, and age-related differences in cognition, behavior, cardiovascular function, the immune system, and metabolism.

Part of a shifting paradigm
Since the 1980s, locus coeruleus neurons were thought to activate synchronously to provide a global neuromodulatory signal across the entire central nervous system. Our lab has contributed to a paradigm shift away from this understanding and toward a new model of locus coeruleus neurophysiology: heterogeneous noradrenergic neuromodulation by a modular locus coeruleus. Our work shows that noradrenergic neurons fire as small collections of neurons called “ensembles.”
Noradrenergic neuromodulation had been thought to drive cortical state away from sleep- and anesthesia-associated “slow waves” ever since Morouzzi and Magoun’s (1949) concept of the Ascending Reticular Activating System (ARAS), through Mircea Steriade’s work on cortico-thalamic loops in the 1990’s, and onward into today’s literature. Our results overturned this textbook view by demonstrating that activation of different locus coeruleus ensembles is associated with distinct cortical states.
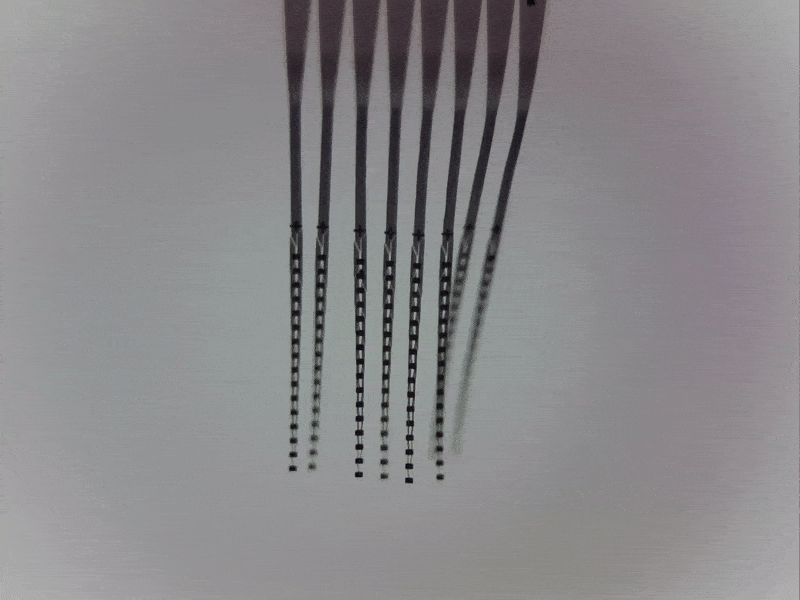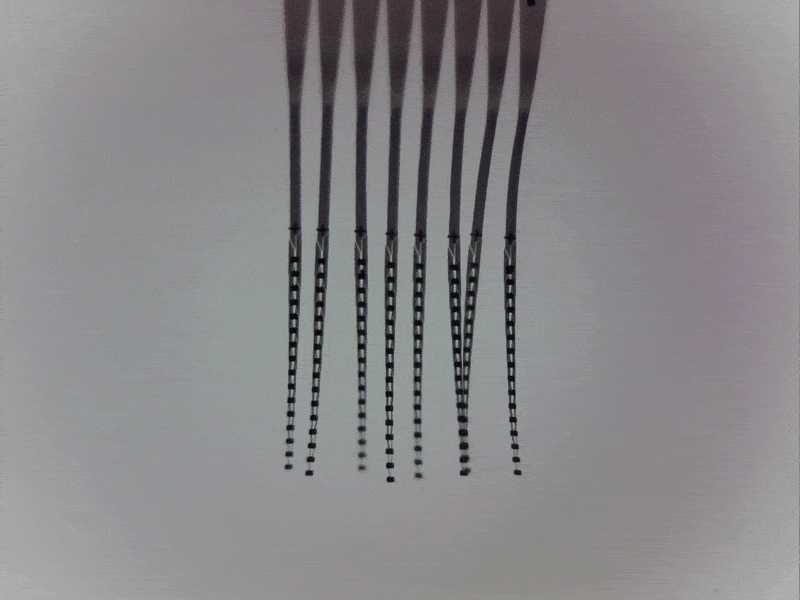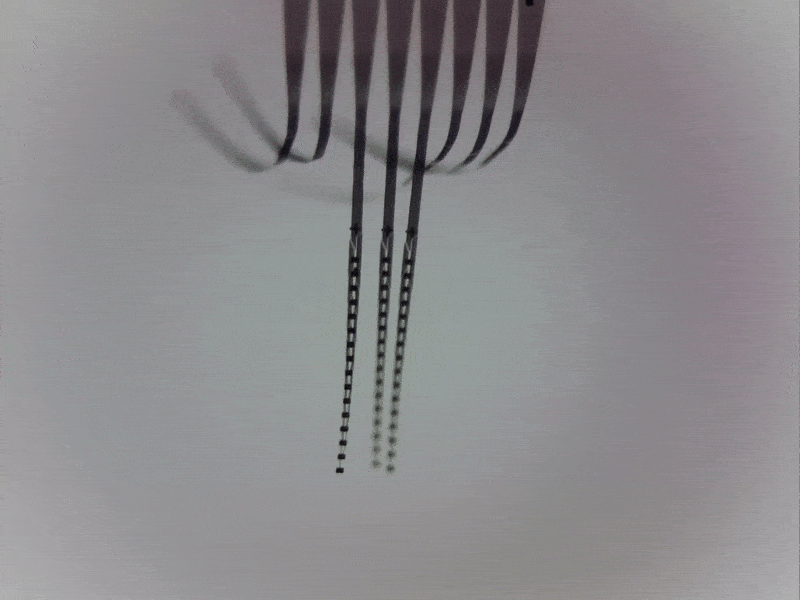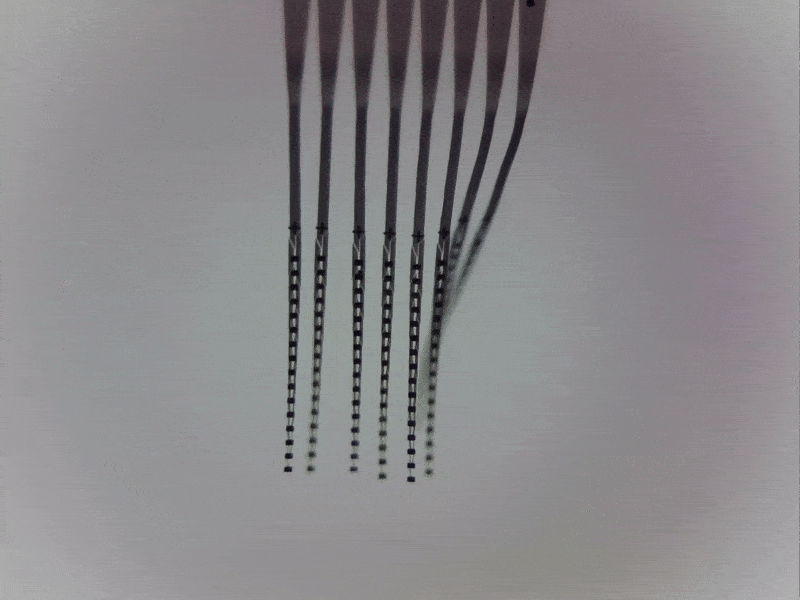
Engineering around a moving target
One of the major challenges in recording locus coeruleus neurons in awake, behaving animals is that the brainstem moves with every body movement, every contraction of the back muscles around the spinal column, and every heartbeat and breath taken by a human or an animal.
Physical instability at the electrode-tissue interface prevents recording more than a few brainstem neurons at once and limits recording times. In collaboration with Prof. Chong Xie at Rice University (Houston, Texas, US), we address this challenge by deploying ultra-flexible multi-electrode “nanoelectronic threads” that can accommodate movement while recording from many neurons simultaneously.




