Research
We aim to discover how neuronal activity creates control over cognition, actions, emotions, and visceral feelings through analysis of behavioral, pupillometry, facial expression, electrocardiogram, and neural data in rats.

The locus coeruleus: an ancestral brainstem region
One of our key targets is the locus coeruleus, a tiny area in the brainstem retained across the vertebrate phylogenetic tree. Evolution has woven this brainstem region deeply into the central nervous system. It influences surprisingly diverse functions in the body and in the mind – from heart rate and immune function to stress response, memory, and beyond.
We are working to solve the mystery of how the activity of locus coeruleus can affect so many diverse functions across a range of vertebrate nervous systems.
How?
Imagine traveling deep down into the brain by steering an electrode the size of a human hair. Your goal: hit a brain region just as small.
That’s what we do.
Because of the minute size of the locus coeruleus and its location deep in the brain, our experiments are like carefully charting a ship’s course in the dark, rock-filled Baltic Sea. We are expert navigators, using electrophysiological landmarks to find our way deep into the brainstem to this precise destination.
Key papers
Diversity of ancestral brainstem noradrenergic neurons across species and multiple biological factors
Kelberman MA, Rodberg E, Arabzadeh E, Bair-Marshall CJ, Berridge CW, Berrocoso E, Breton-Provencher V, Chandler DJ, Che A, Davy O, Devilbiss DM, Downs AM, Drummond G, Dvorkin R, Fazlali Z, Froemke RC, Glennon E, Gold JI, Ito H, Jiang X, Johansen JP, Kaye AP, Kim JR, Kuo CC, Liu RJ, Liu Y, Llorca-Torralba M, McCall JG, McElligott ZA, McKinney AM, Miguelez C, Min MY, Nowlan AC, Omrani M, Poe GR, Pickering AE, Ranjbar-Slamloo Y, Razquin J, Rodenkirch C, Sales AC, Satyasambit R, Shea SD, Sur M, Tkaczynski JA, Torres-Sanchez S, Uematsu A, Vazquez CR, Vreven A, Wang Q, Waterhouse BD, Yang HW, Yang JH, Zhao L, Zouridis IS, Weinshenker D, Vazey E, Totah NK
bioRxiv. 2024.
In search of the locus coeruleus: Guidelines for identifying anatomical boundaries and electrophysiological properties of the blue spot in mice, fish, finches, and beyond
Vreven A, Aston-Jones G, Pickering AE, Poe GR, Waterhouse B, Totah NK
Journal of Neurophysiology. 2024.
Distinct ensembles in the noradrenergic locus coeruleus are associated with diverse cortical states
Noei S, Zouridis IS, Logothetis NK, Panzeri S, Totah NK
Proceedings of the National Academy of Sciences. 2022.
The locus coeruleus is a complex and differentiated neuromodulatory system
Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O
Neuron. 2018.

Navigating free will: how we change our minds
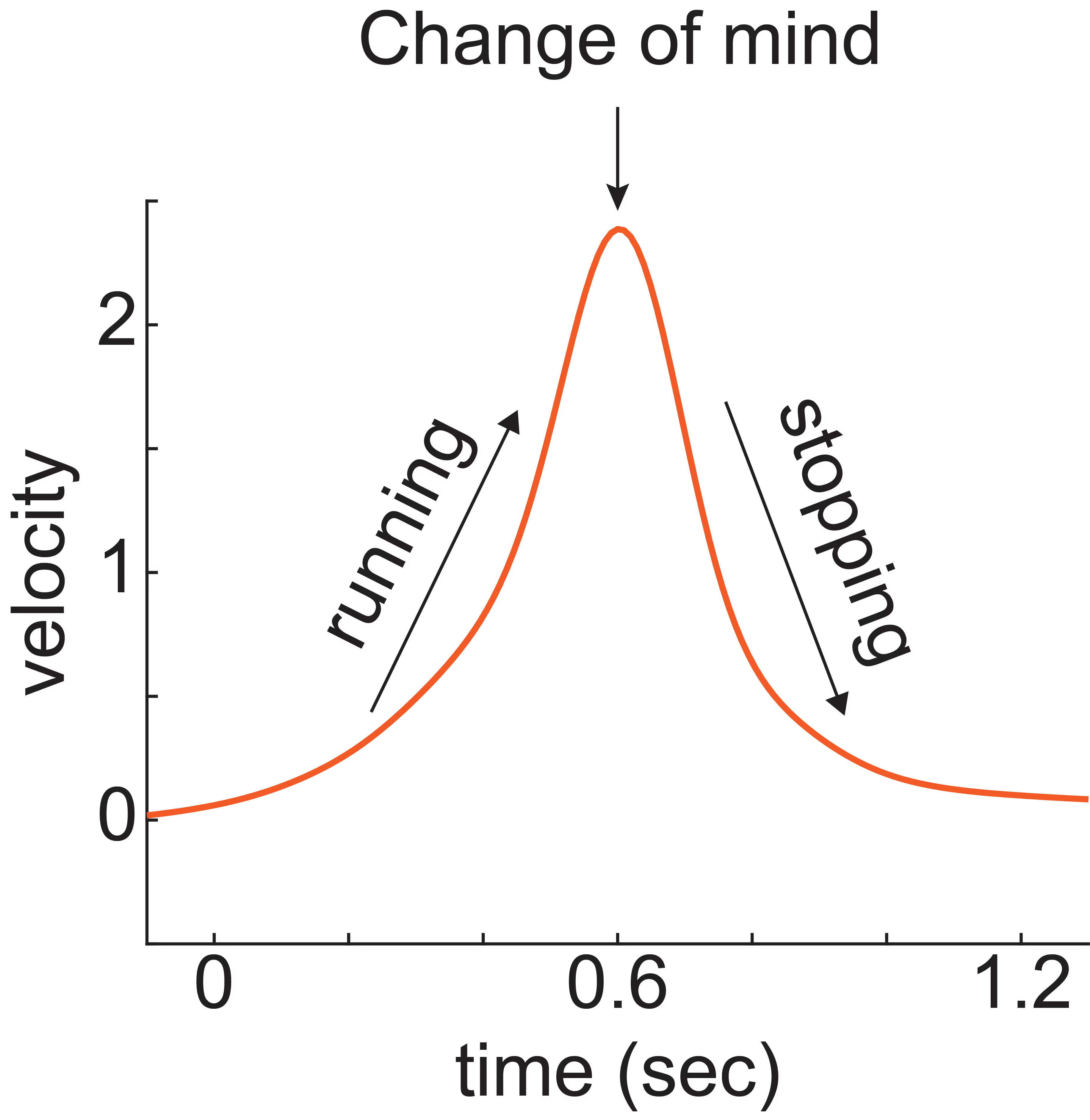
Imagine writing an email when you are angry. As you reach toward your phone to touch “send,” you realize sending it would be a mistake; you change your mind and stop yourself from touching the screen. “Free will” is a topic of lively philosophical inquiry but has limited grounding in physical observations.
We observe this same phenomenon in rats during a pioneering change-of-mind task. The task is an entry point to identify the neural mechanisms underlying free will.
How?
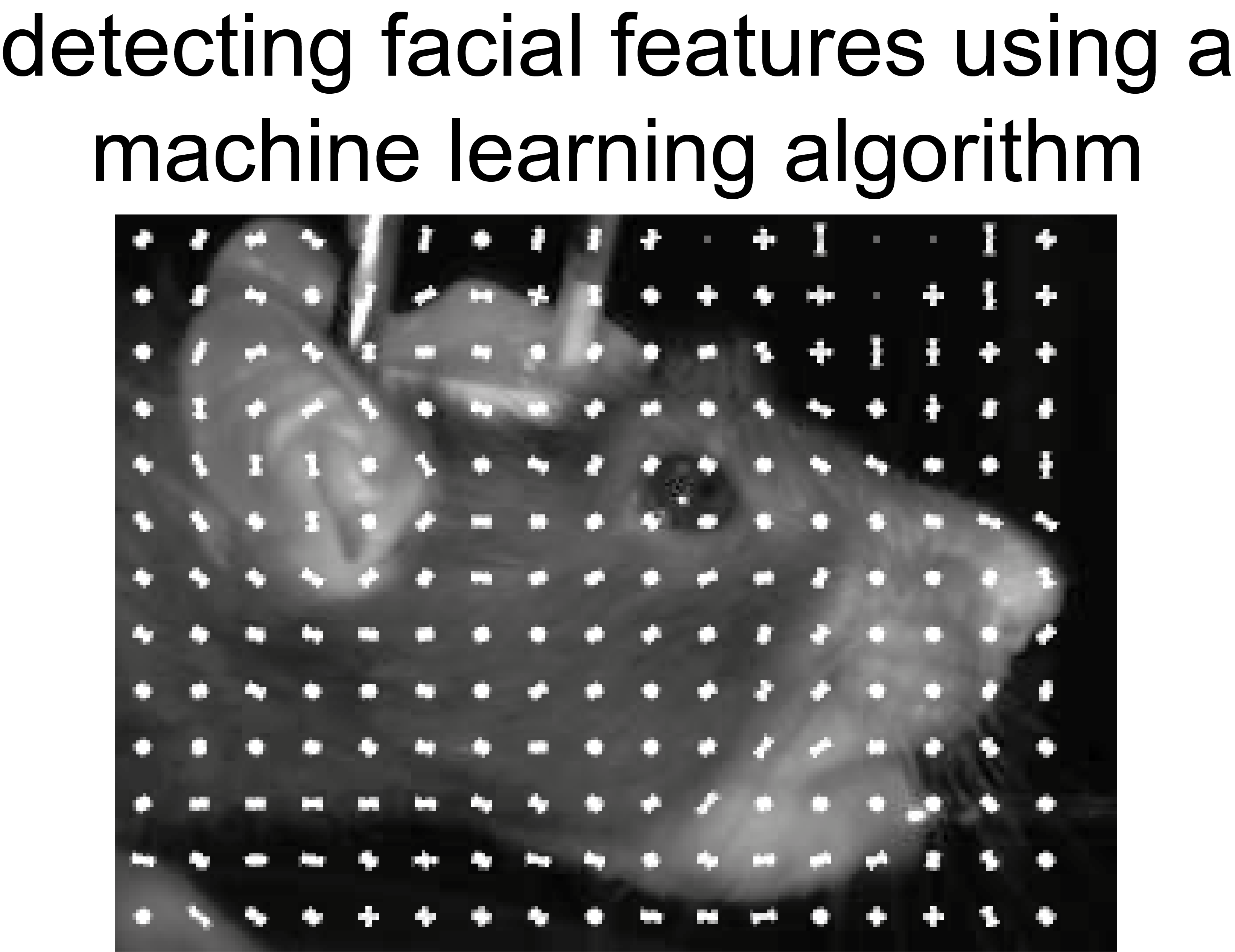
We train rats to run when they see one visual stimulus, and to sit still when they see another visual stimulus. We observed that rats sometimes mistakenly start running, and then realize their mistake and stop running. We are studying brain activity during such changes-of-mind. We have licenced this behavioral technology platform to 3D Neuro and welcome opportunities to collaborate and disseminate this method. (See image 1)
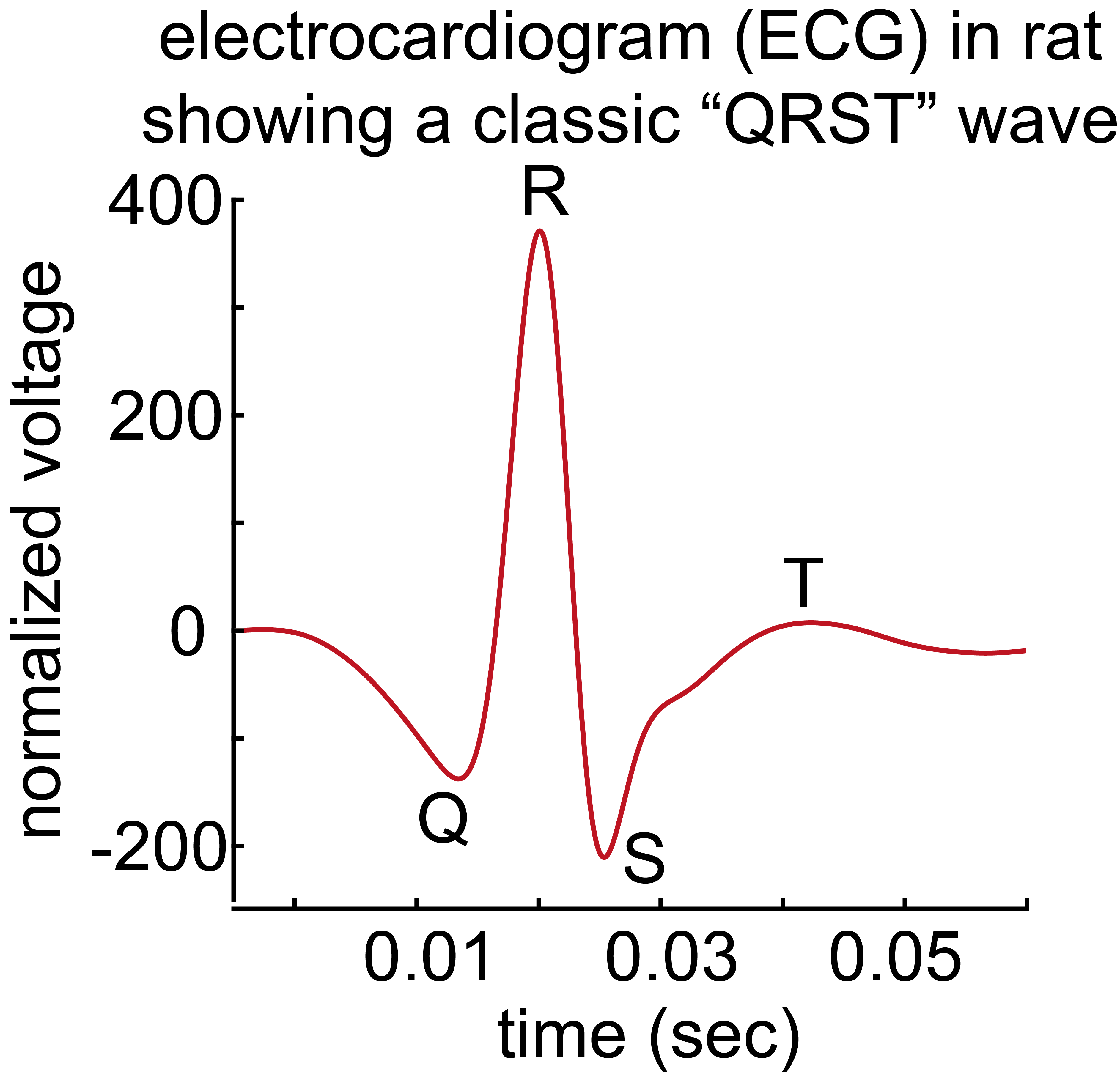
When we catch ourselves from making a mistake, we usually feel badly in a noticeably visceral way: our heart rate changes, we flush, and perhaps make a facial expression (e.g., a grimace). We are investigating this mind-body connection by recording brain activity, electrocardiogram (ECG), and facial expressions when rats catch themselves in a mistake. (See image 2)
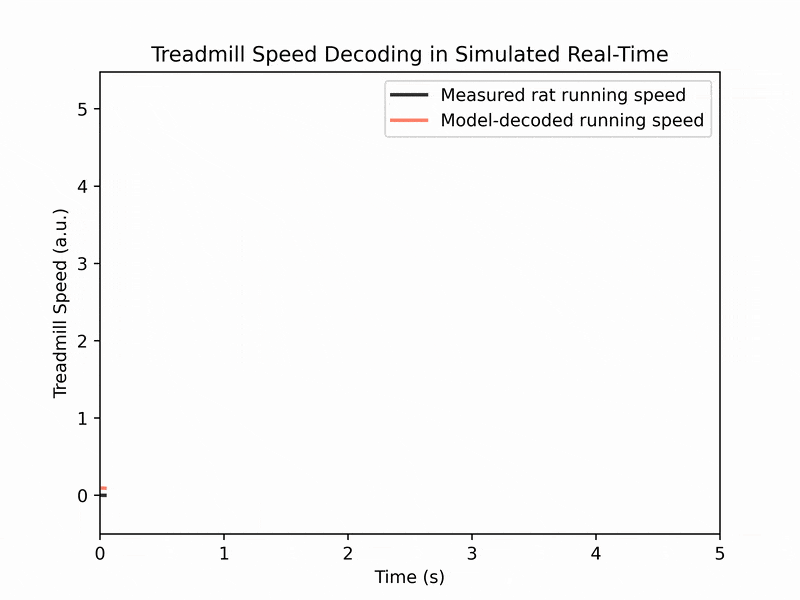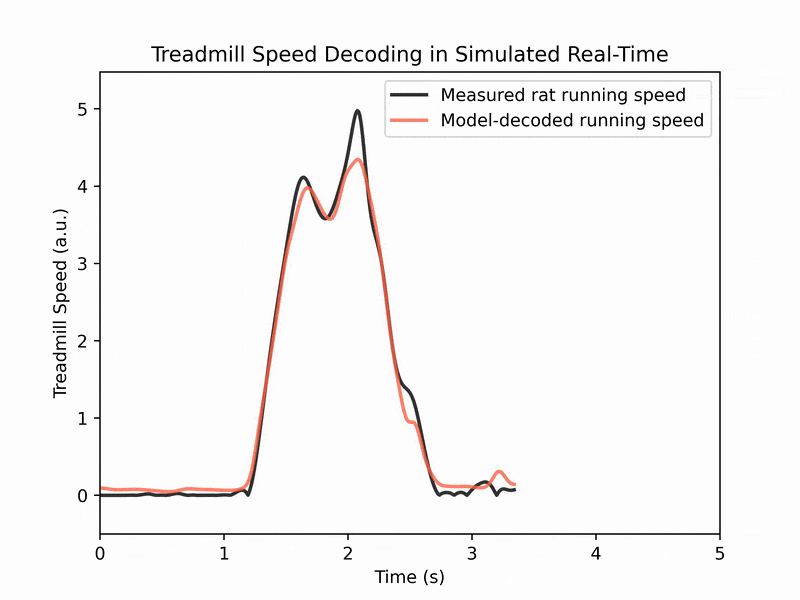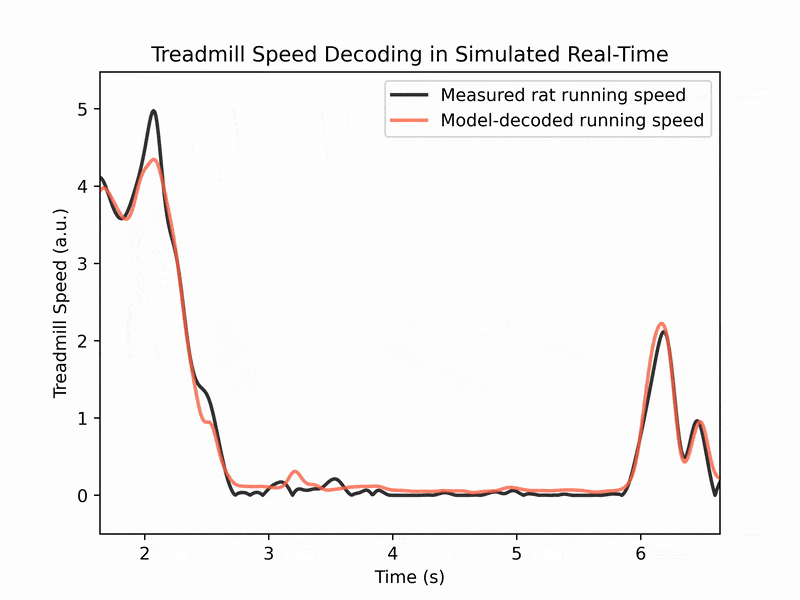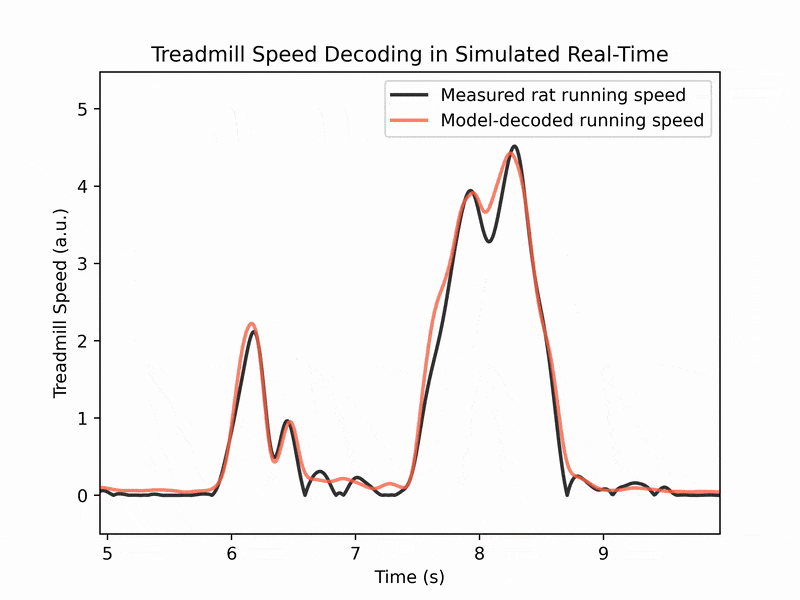
We also collaborate with machine learning labs to perform real-time decoding of running speed from brain activity, which has applications for brain-machine interfaces. (See image 3)
Key papers
Volitional stopping is preceded by a transient beta oscillation
Figueira JFD, Ojala RA, Vasilev D, De Miguel A, Jeay-Bizot L, Iwai R, Raposo I, Safaei N, de Sardenberg Schmid L, Maoz U, Watanabe M, Totah NK
bioRxiv. 2024.
Aberrant prefrontal activity and arousal level correlate with action initiation and response vigor
Klein FJ, Vasilev D, Iwai R, Watanabe M, Totah NK
bioRxiv. 2024.

Neurons to knowledge to AI: how biological neuronal networks let us adapt to a dynamic, multi-sensory world
Adapting our behavior to new rules and concepts is a cornerstone of intelligence. Jean Piaget, the famous educational psychologist, purportedly defined intelligence as “what you use when you don't know what to do: when neither innateness nor learning has prepared you for the particular situation.”
We train rats to solve problems that require them to use this definition of intelligence.
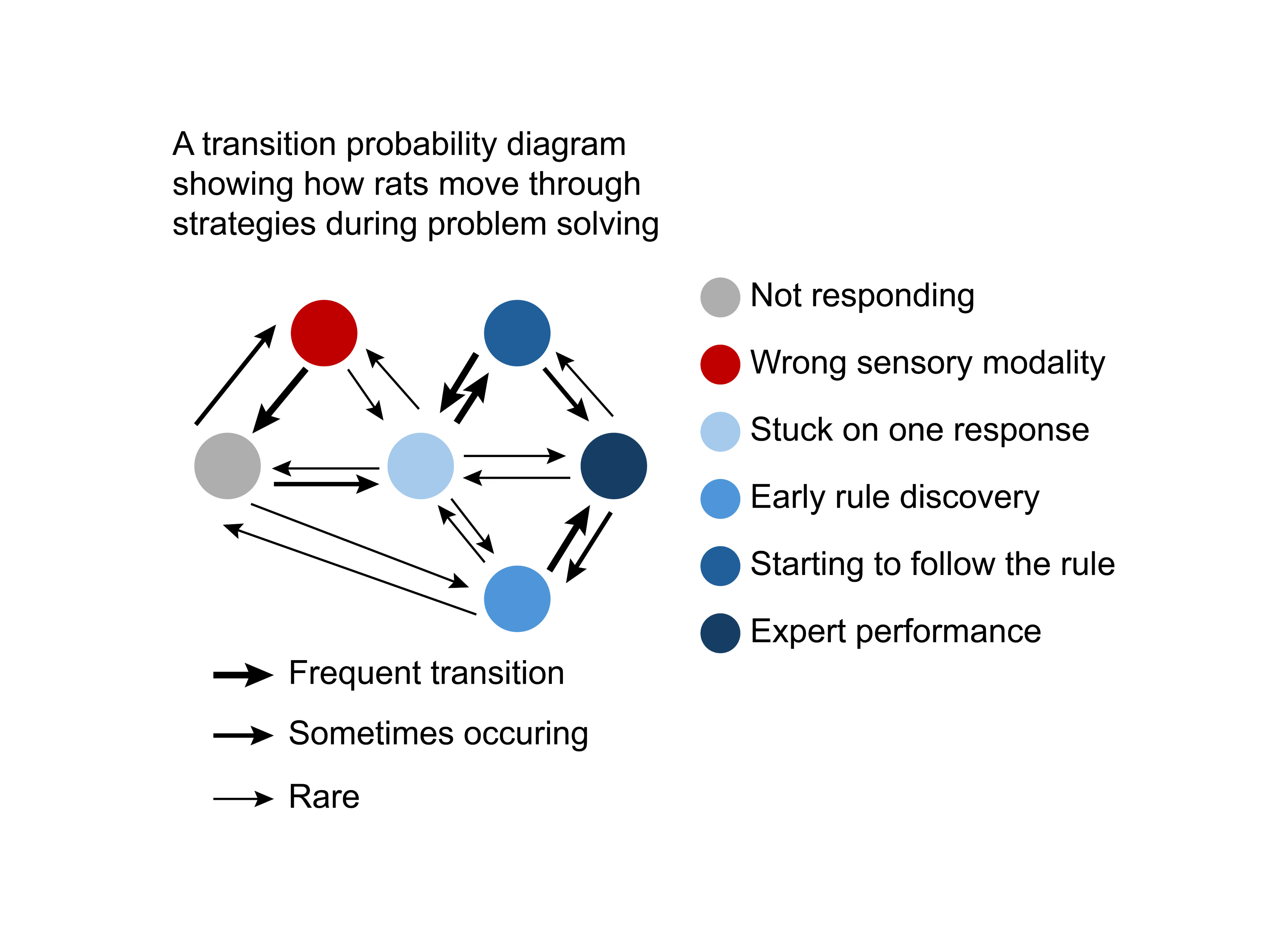
We present rats with simultaneous sights and sounds through which they must learn a visual rule to succeed in their problem-solving task. We then ask them to adapt and learn about a new auditory rule. Through analysis of these rats’ learning strategies and adaptation, we can study the neural basis for flexible, intelligent behavior. Our discoveries about the neural dynamics responsible for this type of intelligence can be extended to shape the algorithms and concepts used in AI.
How?
Using a head-fixed treadmill setup, we introduce rats to visual and audio cues, each linked to specific behaviors. Initially, rats learn a rule based on visual cues—run to one cue, do not run to the other cue — all while ignoring sounds. Then, we switch the game, requiring them to learn to use auditory cues and disregard visual ones. We apply computational tools like Principal Component Analysis (PCA) and Hierarchical Hidden Markov Models to uncover the hidden behavioral strategies that rats use to solve this task.
We are testing the hypothesis that neuromodulatory systems promote the reorganization of cortical neuronal networks that enable the rat to explore the auditory world after becoming biased toward using the visual world to obtain rewards. We use pupillometry to record pupil size as a non-invasive measure of neuromodulatory system activity. Using 32-electrode, whole-cortex EEG recordings of field potentials, signal processing methods, and information theory, we study how interactions between brain regions change as rats discover new rules requiring switching to a sensory modality irrelevant to their earlier rule. We are also testing how computational models of learning - that form the “engine under the hood” of AI - can match the natural, biological intelligence of rats in this task.
Key papers
Focusing perceptual attention in one modality constrains subsequent learning in another modality
Dmitrii Vasilev, Negar Safaei, Ryo Iwai, Hessam Bahmani, Ioannis S. Zouridis, Masataka Watanabe, View ORCID ProfileNikos K. Logothetis, View ORCID ProfileNelson K. Totah
bioRxiv. 2023.

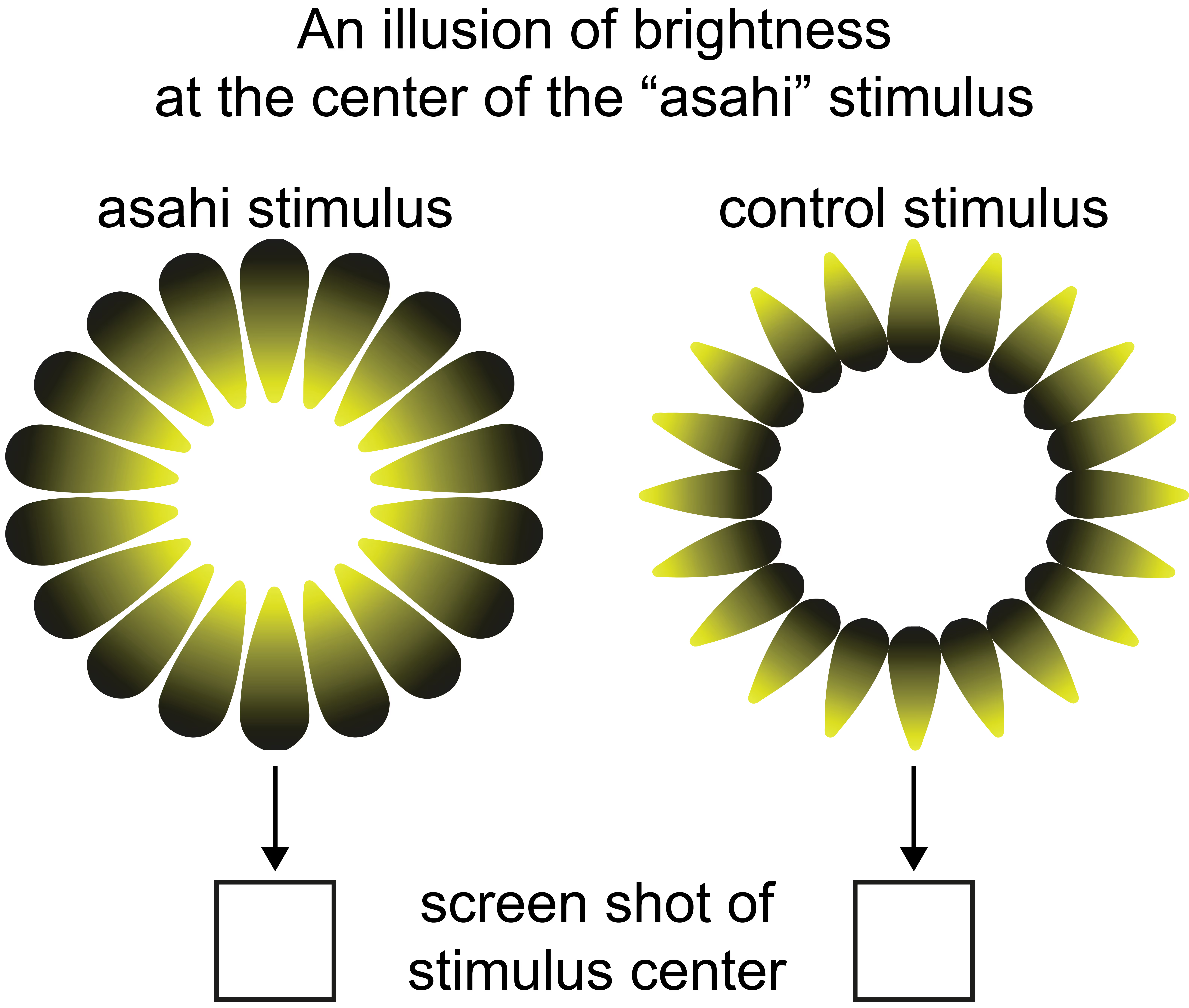
Mind-body connection: the neural link between subjective experiences and physiological responses
In humans, mere illusions of brightness evoke pupil constriction, providing a perfect example of how subjective perceptions can result in real bodily responses. We investigated the neural mechanisms behind this relationship in rats and mice. Our findings support the idea that subjective perception can affect the body (in this case, the eye’s pupil) via primary visual cortex neuronal activity.
How?
We created a new rat behavior paradigm showcasing how the subjective perception of brightness illusions causes constriction of the eye’s pupil. We used 32-electrode EEG to record primary visual cortex neuronal activity changes before pupil constriction.
We collaborated in subsequent work led by Prof. Masataka Watanabe and his lab at the University of Tokyo to replicate the effect of brightness illusions on pupil size in mice. We analyzed primary visual cortex single neuron activity to show those single visual cortex neurons, which are tuned to real line orientations, are also responsive to identical orientations of illusory lines. Using optogenetic inhibition, we demonstrate that this response depends on top-down input from higher visual regions.
Key papers
Brightness illusions drive a neuronal response in the primary visual cortex under top-down modulation
Saeedi A, Wang K, Nikpourian G, Bartels A, Logothetis NK, Totah NK, Watanabe M
Nature Communications. 2024.
Brightness illusions evoke pupil constriction preceded by a primary visual cortex response in rats
Vasilev D, Raposo I, Totah NK
Cerebral Cortex. 2023.